Safety, efficacy, and determinants of allogeneic CAR-NK cell therapy for tumors: a phase 1/2 trial
CAR-T cell therapy marks the advent of the era of cell therapy. However, CAR-T cells have their limitations, including treatment costs, manufacturing time, and toxicities such as cytokine release syndrome (CRS) and neurotoxicity. Therefore, researchers are increasingly paying attention to more cost-effective, safe and effective "off-the-shelf" cell therapies . Natural killer (NK) cells are an indispensable part of the body's anti-cancer defense line. Compared with CAR-T cell therapy , CAR-NK cells retain the intrinsic ability to recognize and target tumor cells through their natural receptors, and can exert a broader spectrum of anti-cancer effects. NK cells do not require strict HLA (human leukocyte antigen) matching and have great potential to be developed into off-the-shelf, universal cell therapies.
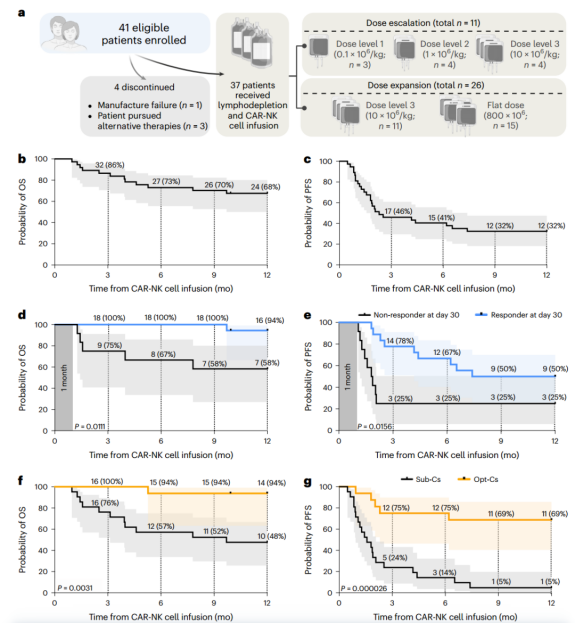
On January 18, 2024, researchers from the University of Texas MD Anderson Cancer Center published a research paper titled "Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial" in Nature Medicine . A phase 1/2 trial of umbilical cord blood-derived NK cells expressing anti-CD19 chimeric antigen receptor and interleukin-15 (CAR19/IL-15) was conducted in 37 patients with CD19+ B cell malignancies.

The authors used retroviral transduction to transduce NK cells from umbilical cord blood to express anti-CD19 CAR, and also expressed IL-15 to enhance the expansion of CAR-NK cells in vivo, and expressed the inducible caspase-9 (iC9) suicide gene, which can induce apoptosis of CAR-NK cells in the event of unacceptable toxicity, thereby increasing its safety. The median transduction efficiency of the final transfused CAR19/IL-15 NK cells was 72.4% (range 22.7-91.1, retroviral vectors have high transfection efficiency in NK cells, and lentiviral vectors have low transfection efficiency, only 20%). The median content of CD3-positive T cells in the transfused product was 2,000 cells/kg (about 2%, ranging from 30-16,000 cells per kilogram of body weight). Shenzhen Cell Valley has currently achieved a major breakthrough in the preparation of NK cells , with a purity of 96% for peripheral blood-derived NK cells and less than 1% for T cells. Moreover, the CD19 CAR-NK prepared based on retroviral vector has a transduction positivity rate of up to 60% to 80%, which can better promote the clinical research of NK and CAR-NK.
The primary goal of this clinical trial is safety and efficacy uation, defined as the overall response rate (OR) at day 30 of treatment. Secondary goals include overall response rate at day 100, progression-free survival (PFS), overall survival (OS), and persistence of CAR19/IL-15 NK cells. The results showed that patients did not experience severe cytokine release syndrome, neurotoxicity, or graft-versus-host disease. The OR rates on days 30 and 100 were both 48.6%. The one-year overall survival rate and progression-free survival rate were 68% and 32%, respectively. Patients who achieved OR had higher levels of CAR-NK cells and longer persistence.
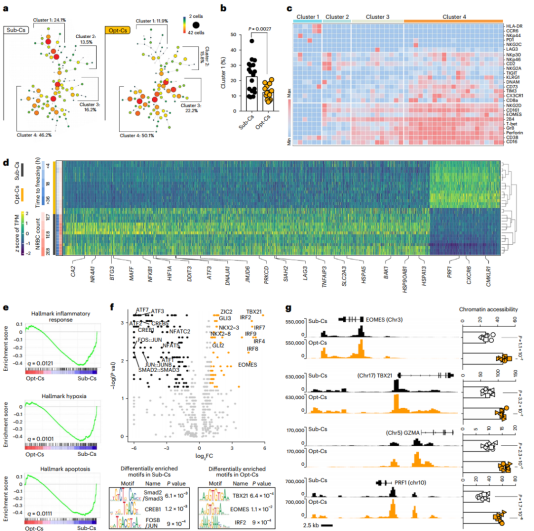
Among other things, the study explored whether cord blood unit (CBU) characteristics impact patient outcomes. Results showed that CAR-NK cells receiving CBU of ≤8 × 10 7 nucleated red blood cells and cryopreserved for ≤24 hours were the most important predictors of excellent outcomes. NK cells derived from these optimal CBUs are highly functional and enriched in effector-related genes. In contrast, NK cells derived from suboptimal CBUs have upregulation of inflammatory, hypoxic, and cellular stress programs. The research team further confirmed in three tumor mouse models of lymphoma, multiple myeloma and ovarian cancer that CAR/IL-15 NK cells from the best CBU have better in vivo proliferation ability, thereby achieving better tumor control. These findings reveal new features of CAR-NK cell biology and highlight the importance of donor selection in allogeneic cell therapy.
Using allogeneic immune cells from healthy donors has several advantages over cells from autologous patients, including generating multiple doses of therapeutic cells from a single donor that can be cryopreserved for off-the-shelf use, making allogeneic products cost-effective Effective, easily accessible, and with the potential for sustained and high-quality results. In summary, this study demonstrates that CAR19/IL-15 CBU-NK cells have similar efficacy to autologous CAR19-T cells, but have a significantly improved safety profile because CAR19/IL-15 CBU-NK cells are associated with significant CRS or neurological deficits. Toxicity is irrelevant. In addition, the findings highlight the importance of allogeneic donor selection criteria for CAR-NK cell production and the identification of predictors of donor-specific response , providing an experimental basis for future donor selection criteria for clinical trials using CBU-derived NK cells. and scientific basis.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people know about the new development of biomedicine. The content of this article is only used for information exchange. This platform is neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information of this article shall not be used for diagnosis or treatment, can not substitute for professional medical opinion, the company website will not bear any responsibility. The final interpretation of the above statement belongs to the company website, this statement will apply to all the time shared articles, thank you for your cooperation! Copyright: The copyright of the article belongs to Shenzhen Cell Valley, and individuals are welcome to forward it to the circle of friends. Media or institutions, who will reprint it to other platforms in any form without authorization, will be regarded as infringement. If you need to reprint, please contact the email address: contact@duanglink.com
On January 18, 2024, researchers from the University of Texas MD Anderson Cancer Center published a research paper titled "Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial" in Nature Medicine . A phase 1/2 trial of umbilical cord blood-derived NK cells expressing anti-CD19 chimeric antigen receptor and interleukin-15 (CAR19/IL-15) was conducted in 37 patients with CD19+ B cell malignancies.

The authors used retroviral transduction to transduce NK cells from umbilical cord blood to express anti-CD19 CAR, and also expressed IL-15 to enhance the expansion of CAR-NK cells in vivo, and expressed the inducible caspase-9 (iC9) suicide gene, which can induce apoptosis of CAR-NK cells in the event of unacceptable toxicity, thereby increasing its safety. The median transduction efficiency of the final transfused CAR19/IL-15 NK cells was 72.4% (range 22.7-91.1, retroviral vectors have high transfection efficiency in NK cells, and lentiviral vectors have low transfection efficiency, only 20%). The median content of CD3-positive T cells in the transfused product was 2,000 cells/kg (about 2%, ranging from 30-16,000 cells per kilogram of body weight). Shenzhen Cell Valley has currently achieved a major breakthrough in the preparation of NK cells , with a purity of 96% for peripheral blood-derived NK cells and less than 1% for T cells. Moreover, the CD19 CAR-NK prepared based on retroviral vector has a transduction positivity rate of up to 60% to 80%, which can better promote the clinical research of NK and CAR-NK.
The primary goal of this clinical trial is safety and efficacy uation, defined as the overall response rate (OR) at day 30 of treatment. Secondary goals include overall response rate at day 100, progression-free survival (PFS), overall survival (OS), and persistence of CAR19/IL-15 NK cells. The results showed that patients did not experience severe cytokine release syndrome, neurotoxicity, or graft-versus-host disease. The OR rates on days 30 and 100 were both 48.6%. The one-year overall survival rate and progression-free survival rate were 68% and 32%, respectively. Patients who achieved OR had higher levels of CAR-NK cells and longer persistence.

Among other things, the study explored whether cord blood unit (CBU) characteristics impact patient outcomes. Results showed that CAR-NK cells receiving CBU of ≤8 × 10 7 nucleated red blood cells and cryopreserved for ≤24 hours were the most important predictors of excellent outcomes. NK cells derived from these optimal CBUs are highly functional and enriched in effector-related genes. In contrast, NK cells derived from suboptimal CBUs have upregulation of inflammatory, hypoxic, and cellular stress programs. The research team further confirmed in three tumor mouse models of lymphoma, multiple myeloma and ovarian cancer that CAR/IL-15 NK cells from the best CBU have better in vivo proliferation ability, thereby achieving better tumor control. These findings reveal new features of CAR-NK cell biology and highlight the importance of donor selection in allogeneic cell therapy.

Using allogeneic immune cells from healthy donors has several advantages over cells from autologous patients, including generating multiple doses of therapeutic cells from a single donor that can be cryopreserved for off-the-shelf use, making allogeneic products cost-effective Effective, easily accessible, and with the potential for sustained and high-quality results. In summary, this study demonstrates that CAR19/IL-15 CBU-NK cells have similar efficacy to autologous CAR19-T cells, but have a significantly improved safety profile because CAR19/IL-15 CBU-NK cells are associated with significant CRS or neurological deficits. Toxicity is irrelevant. In addition, the findings highlight the importance of allogeneic donor selection criteria for CAR-NK cell production and the identification of predictors of donor-specific response , providing an experimental basis for future donor selection criteria for clinical trials using CBU-derived NK cells. and scientific basis.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people know about the new development of biomedicine. The content of this article is only used for information exchange. This platform is neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information of this article shall not be used for diagnosis or treatment, can not substitute for professional medical opinion, the company website will not bear any responsibility. The final interpretation of the above statement belongs to the company website, this statement will apply to all the time shared articles, thank you for your cooperation! Copyright: The copyright of the article belongs to Shenzhen Cell Valley, and individuals are welcome to forward it to the circle of friends. Media or institutions, who will reprint it to other platforms in any form without authorization, will be regarded as infringement. If you need to reprint, please contact the email address: contact@duanglink.com



